Kate gave talk at the Fargo Theater on engineering synthetic life.

new publication
|
|
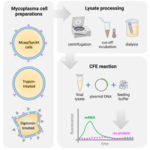
Traditional protocols and optimization methods lead to absent expression in mycoplasma cell-free gene expression platform |
The life of our Last Universal Common Ancestor
Synthetic biology allows us to build minimal cell-like systems, to investigate origin of life and build tools for space exploration.
The life of our Last Universal Common Ancestor | LAS 2021.
new publication
|
|
Akaby – cell-free protein expression system for linear templates |
new publication
|
|
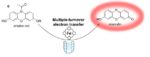
Diffusion control in biochemical specificity; |
new publication
|
|
Switchable DNA-based Peroxidases Controlled by a Chaotropic Ion; |
Build-a-Cell workshop
Abbey, Anders and Orion presented posters at Build-a-Cell Workshop 8 at Caltech.

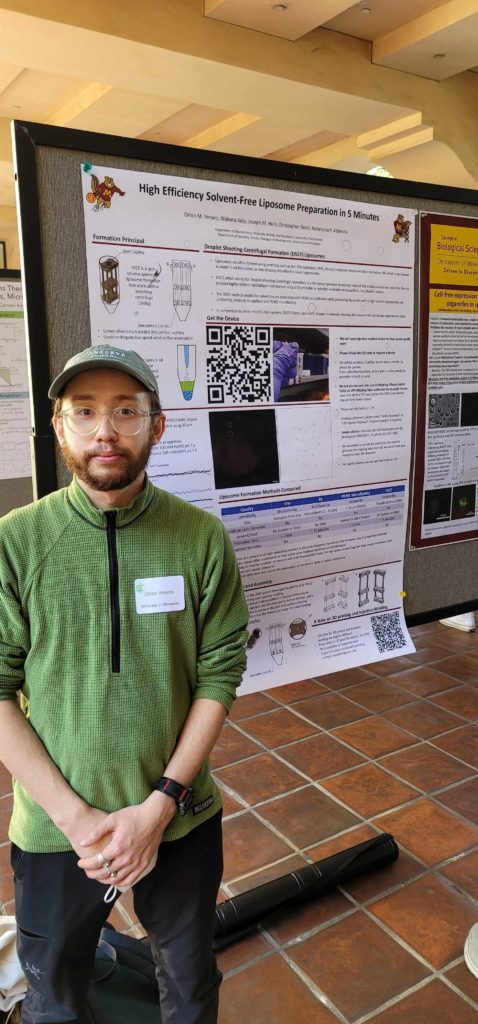
new publication
|
|
Making Security Viral: Shifting Engineering Biology Culture and Publishing; |
Akaby
Akaby strain for linear TxTl is now available to all non-profit users: Akaby strain and information

new publication
|
|
Liposome Preparation by 3D-Printed Microcapillary-Based Apparatus; |